By David W. Brown
Glutathione (GSH) is referred to as the “super antioxidant” because of its central role in protecting and regulatingnearly every cell in the human body. This small but powerful tripeptide is crucial for defending against oxidative stress, detoxifying harmful compounds, modulating immune activity, and supporting mitochondrial energy production. Without adequate glutathione, cellular health and survival are significantly compromised.
Chemically, glutathione is simple, but biologically it is indispensable. Its sulfur chemistry enables electron transfer reactions that protect DNA, proteins, and membranes from damage. Understanding its structure, biosynthesis, and pathways reveals why glutathione is so vital for health.
Chemistry of Glutathione
Glutathione is a tripeptide, composed of three amino acids:
- Glutamate
- Cysteine (the critical sulfur donor)
- Glycine
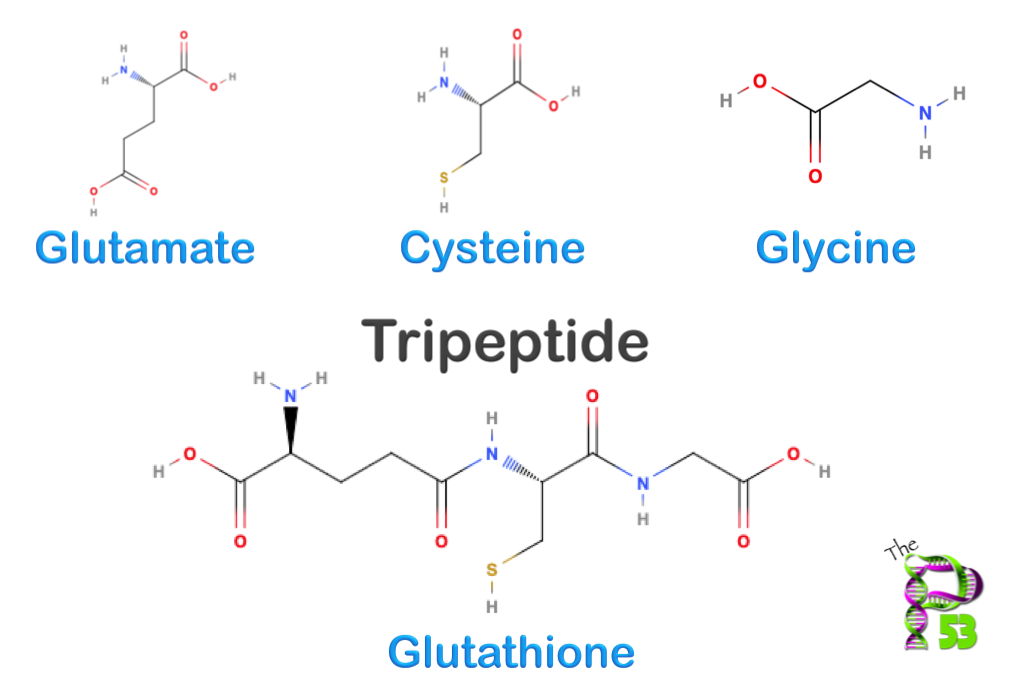
Its uniqueness lies in the γ-glutamyl bond connecting glutamate to cysteine. This unusual bond makes glutathione resistant to most proteases, giving it stability inside cells.
The cysteine component carries a thiol (-SH) group, the chemically reactive part of the molecule. This sulfhydryl group can donate electrons, neutralizing reactive oxygen species (ROS) and other oxidants.
Glutathione exists in two major forms:
- Reduced glutathione (GSH) – the active antioxidant
- Oxidized glutathione (GSSG) – formed when two GSH molecules join via a disulfide bond
The GSH:GSSG ratio is a critical biomarker of oxidative stress. Healthy cells maintain a high ratio of reduced glutathione, while a shift toward oxidized glutathione signals cellular damage.
Glutathione Biosynthesis
Glutathione is synthesized inside cells through a two-step, ATP-dependent pathway:
- γ-Glutamylcysteine synthetase (glutamate-cysteine ligase) joins glutamate and cysteine.
- This is the rate-limiting step.
- Cysteine availability is the main bottleneck since dietary cysteine is less abundant.
- Glutathione synthetase adds glycine to complete the tripeptide.
Because ATP is required, glutathione production is tied to energy status. In times of oxidative stress, expression of γ-glutamylcysteine synthetase is boosted through the Nrf2 antioxidant response pathway, increasing glutathione synthesis.
Pathways Involving Glutathione
1. Redox Regulation and ROS Neutralization
Glutathione directly controls oxidative stress through the glutathione peroxidase (GPx) system:
- GPx reduces hydrogen peroxide (H₂O₂) and lipid peroxides by using GSH as an electron donor.
- GSH is oxidized to GSSG in the process.
- Glutathione reductase then recycles GSSG back to GSH using NADPH.
This cycle prevents lipid peroxidation, protein oxidation, and DNA strand breaks. It is one of the most important antioxidant defenses in biology.
2. Detoxification and Phase II Conjugation
Glutathione plays a leading role in detoxification through glutathione S-transferase (GST) enzymes:
- GST attaches glutathione to electrophilic toxins, drugs, and carcinogens.
- This conjugation makes the compounds water-soluble so they can be excreted in bile or urine.
- Heavy metals, pesticides, and pollutants are often cleared this way.
A well-known clinical example is acetaminophen overdose. When the liver runs out of glutathione to detoxify acetaminophen’s reactive metabolite (NAPQI), the toxin binds to proteins and causes severe liver damage.
3. Mitochondrial Protection
Mitochondria are both the main source of cellular energy and the main source of reactive oxygen species. Glutathione is the primary mitochondrial antioxidant:
- It preserves mitochondrial DNA from oxidative mutations.
- It protects the respiratory chain enzymes from thiol oxidation.
- It maintains the mitochondrial membrane potential necessary for ATP production.
Without mitochondrial glutathione, cells are vulnerable to apoptosis or necrosis due to energy failure.
4. Immune Function and Inflammation Control
Glutathione is critical for a balanced immune system:
- It supports T-cell and natural killer cell activity.
- It regulates antigen presentation in immune cells.
- It influences cytokine release, preventing both deficiency and excessive inflammation.
When glutathione levels drop, immune responses can become impaired or dysregulated, leading to either weakened defense or chronic inflammation.
5. Nitric Oxide and Vascular Function
Glutathione interacts with nitric oxide (NO) to form S-nitrosoglutathione (GSNO), which regulates NO bioavailability:
- Helps maintain blood vessel dilation and normal blood pressure.
- Prevents inappropriate platelet aggregation.
- Supports endothelial cell health and vascular flexibility.
Disruption of this system contributes to hypertension and cardiovascular disease.
6. Protein Folding and Redox Signaling
In the endoplasmic reticulum (ER), glutathione contributes to protein folding by regulating disulfide bond formation. It also modifies proteins through S-glutathionylation, a reversible reaction attaching glutathione to cysteine residues on enzymes and receptors.
This modification fine-tunes signaling pathways, controlling cell proliferation, apoptosis, and stress responses.
Glutathione and Human Health
Because of its central role, glutathione depletion or imbalance is linked to many conditions:
- Neurodegenerative disorders: Parkinson’s, Alzheimer’s, and ALS all show reduced glutathione levels in affected brain regions, leading to increased oxidative stress and neuron loss.
- Cancer: Glutathione helps protect DNA from mutations, but cancer cells often hijack glutathione pathways to resist chemotherapy.
- Cardiovascular disease: Low glutathione contributes to endothelial dysfunction, arterial plaque formation, and impaired nitric oxide signaling.
- Aging: The glutathione pool declines naturally with age, weakening antioxidant defenses and increasing susceptibility to chronic illness.
- Liver disease: Alcohol, drugs, and toxins place a heavy demand on liver glutathione. Chronic depletion contributes to fatty liver, fibrosis, and cirrhosis.
Supporting Glutathione Levels
Since cysteine is the rate-limiting factor in glutathione synthesis, dietary intake of sulfur-containing nutrients is critical. Ways to support healthy glutathione include:
- Foods rich in sulfur compounds: garlic, onions, leeks, and cruciferous vegetables (broccoli, kale, Brussels sprouts).
- Plant protein sources of methionine and cysteine: lentils, sunflower seeds, oats, beans.
- Cofactors for glutathione recycling:
- Vitamin C (recycles oxidized glutathione)
- Vitamin E (works synergistically with GSH)
- Selenium (essential for glutathione peroxidase function)
- Alpha-lipoic acid (enhances glutathione regeneration)
Lifestyle choices also influence glutathione status. Chronic stress, environmental toxins, alcohol use, smoking, and poor diet all deplete glutathione reserves.
Glutathione is far more than a simple antioxidant. It is a metabolic hub that integrates redox regulation, detoxification, mitochondrial protection, immune balance, vascular health, and protein signaling. Its unique sulfur chemistry gives it unparalleled ability to protect the body against oxidative and toxic damage.
Declining glutathione is strongly associated with aging and chronic disease, while optimal levels support resilience, energy, and long-term health. Maintaining glutathione through proper nutrition, lifestyle, and metabolic support is one of the most powerful ways to safeguard cellular health.
